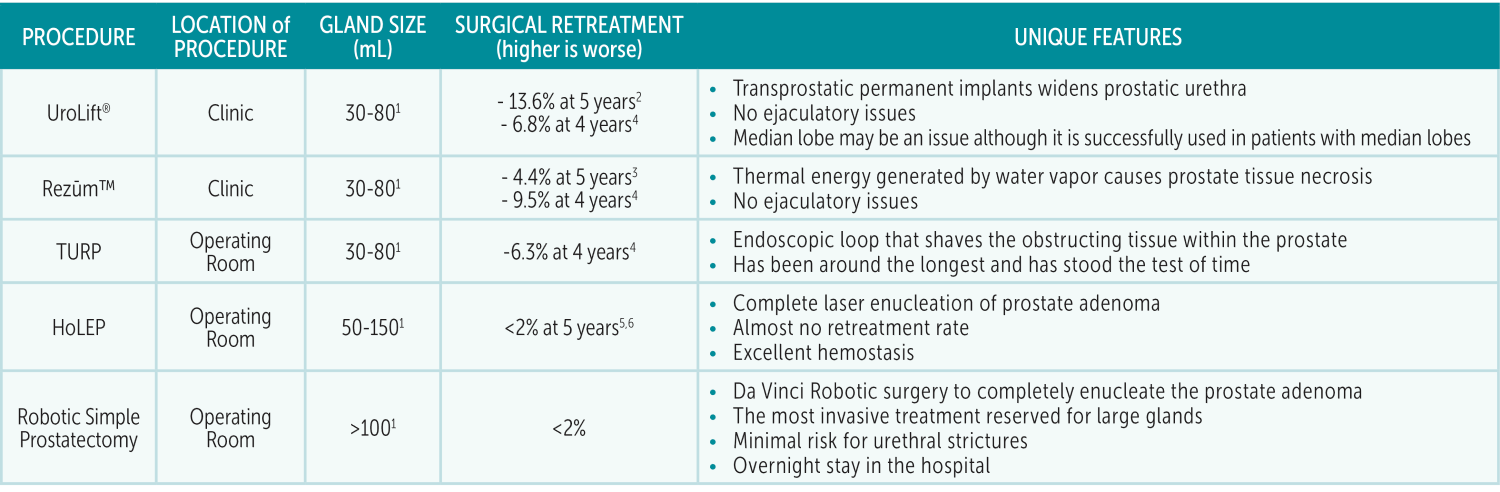
Benign prostatic hyperplasia/enlargement (BPH/BPE) is a common diagnosis among aging men. Men with persistent symptoms despite medications or those who cannot tolerate medications will benefit from procedures. In the current era, there are multiple options available: Rezūm™, UroLift®, Transurethral Resection of Prostate (TURP), Greenlight Photovaporization of Prostate (PVP), Holmium Laser Enucleation (HoLEP), and Robotic Simple Prostatectomy. With so many options available, how do you know which one is right for your patient?
UroLift® was approved by the FDA in 2013. This technology uses clips that compress the prostatic urethra to allow a bigger channel for outflow. It is also approved for treatment of glands up to 80mL. It is performed in the office with local anesthesia around the prostate with the option for nitrous oxide for additional sedation. The procedure can also be performed in the clinic with local anesthesia around the prostate. Typically, a post-procedure Foley catheter for UroLift® is not needed.
Rezūm™ Therapy was approved by the FDA in 2015. The technology works by delivering a small amount of steam to the prostate. This destroys a small amount of obstructing prostate tissue with each injection. A Foley catheter is left in for approximately three days after the procedure. It is approved for treatment of glands up to 80mL.
As for endoscopic treatments for BPH, TURP has been around the longest. It requires a trip to the operating room under general anesthesia. The prostate is then resected from the inside with a tiny cautery loop under direct magnified vision. A catheter is left in for a few days after the procedure and patients can often discharge the same day, although some patients may need to stay overnight. Another endoscopic procedure is Greenlight PVP. Rather than using a loop current to resect the tissue, a laser is used to ablate or vaporize the tissue. A catheter is also often left in and patients can discharge the same day. The advantage of Greenlight PVP is improved hemostasis compared to TURP.
HoLEP was developed in the 1990s. It is a unique treatment in that a laser is used to separate the prostate obstructing adenoma from the outer shell of the prostate. It requires a trip to the operating room under general anesthesia. A catheter is left in for at least one day and many patients can go home the same day. The benefit of this procedure is the complete removal of the prostate adenoma, improved hemostasis, and exceptionally low lifetime re-treatment rates (<2%). There can be expected post-operative leakage of urine; however, these symptoms usually resolve by 6 weeks. This procedure can be challenging to master, which has limited the number of surgeons who perform this procedure. Often times urologists need to do a fellowship in order to be comfortable with this procedure. I was lucky enough to have had training in this technique during my fellowship at Beth Israel Deaconess Medical Center in Boston.
UroLift® and Rezūm™ are considered MIST (minimally invasive surgical therapy). They have never been studied directly against each other in a clinical trial; therefore, it is challenging to determine if one is more effective than the other. Nonetheless, the advantage of MIST is the ability to be performed in the clinic without general anesthesia and they do not cause any ejaculatory side effects. Although there are minimal to no effects on orgasms and erections with any of the BPH procedures, TURP, HoLEP and Simple prostatectomy can cause retrograde ejaculation. The advantages of UroLift® and Rezūm™ are offset by the fact that there is a significantly higher retreatment rate five years after the procedure compared to HoLEP and Robotic Simple Prostatectomy. HoLEP and Robotic Simple Prostatectomy are considered definitive therapy with extremely low re-treatment rates. Each procedure has its place in the armamentarium for BPH treatment and the procedure should be tailored to the patient’s anatomy and preferences.
References
1. Lerner et al. AUA Guideline Part II. J Urol 2021;206:818.
2. Roehrborn et al. Can J Urol. 2017;24(3):8802-13
3. McVary et al. J Urol. 2020;203(4):e1021
4. Kaplan et al. Analysis of Real-world Healthcare Claims. EAU Conference Presentation. 2021.
5. Elmansy et al. J Urol. 2011;186:1972
6. Krambeck et al. J Urol. 2013;189:S141